Use QRM rules to ascertain cleaning validation requirements when employing committed gear or amenities. Regions of concern involve:
Make certain that tools and facility style and design, operation, cleaning and routine maintenance will appropriately control microbiological bioburden. Give attention to preventative steps rather then removing of contamination after it has happened.
The schedule or periodic sampling system have to enable the maker to watch essential cleaning attributes whilst minimally impacting the cleaning turnaround time. As an example, certain analytical methods including superior-effectiveness liquid chromatography (HPLC) are most popular for validation applications, Whilst nonspecific methods including conductivity, titration, or full organic carbon (TOC) could possibly be extra ideal for program use due to their quickly response moments.
Be certain that closing rinse/sample rinse and machines are free in the characteristic odor in the preceding products shall be confirmed via the smelling of cleaned machines part.
The Selection of solvent for any swab, if besides water shall be determined by the solubility in the active ingredient.
Chemical Criterion: no more than ten ppm (pieces for each million) of a product ought to be detected in another product and/or not more than 0.1% of the conventional therapeutic dose of a product should look in the most every day dose of Yet another products
Phase 3 - On-likely checking: Ensure cleaning strategies remain powerful and controlled by means of an ongoing monitoring application.
Coaching shall be provided by subjecting officers to assessment and determine the drug material residue at a lessen level which happens to be created by recognizing answers of lessen focus (at LOD stage) on all MOC associated with devices cleaning, performed in the course of recovery scientific tests carried out because of the laboratory for method validation of your analytical method.
Cleaning methods which have been effective at obtaining better boundaries than those derived from HBELs need to continue to take action. Take note that cleaning boundaries will have to also keep on to fulfill the visually thoroughly clean standards.
All new solution introductions needs to be reviewed throughout the QRM procedure and alter Command to ascertain irrespective of whether the existing technological and organizational controls are enough or have to be modified. Contemplate the following:
Exactly the same method shall be applicable for that specific product throughout schedule cleaning things to do after the successful completion of cleaning validation.
Efficient cleaning validation can reduce excellent fees, click here keep item integrity, and increase client safety. Listed down below are 3 very simple guideline inquiries to assist quality assurance and output departments layout cleaning validation protocols correctly:
Visual inspection can be a qualitative method of assessing products cleanliness and includes verifying that devices is freed from noticeable residue and foreign material at merchandise changeover.
This template is used to complete the process validation protocol by reporting the verification in the devices/procedure final style towards website the consumer, useful, and/or style and design requirements. Conveniently identify important gear elements, utilities provide, and environmental needs.
 Judd Nelson Then & Now!
Judd Nelson Then & Now! Robert Downey Jr. Then & Now!
Robert Downey Jr. Then & Now! Alexa Vega Then & Now!
Alexa Vega Then & Now!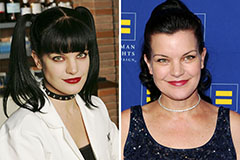 Pauley Perrette Then & Now!
Pauley Perrette Then & Now! Samantha Fox Then & Now!
Samantha Fox Then & Now!